About
DAEUN MEDICAL

Founded in 2010, Daeun Medical is a medical device manufacturing company that develops and produces
wound treatment materials based on its own “wound treatment composition” technology. We are striving to
develop products that can be applied to various areas in the future and to explore markets in various
countries.

Mission & Vision
Mission
To contribute to healthy and wellness of mankind with safe technologyVision
Innovated HealthcareCore Value
User Value
First
Develop products and services
that prioritize user value
based on empathy, respect and dignity
First
Market
Leader
First Mover,
Who Leads the Market Change
Leader
Rational
Management
Rational decision making
based on high standards of ethics
and social responsibility
Management
Be
Global
Sharing advanced values
to mankind around the world
Global
History
2025
- Expected to export to Turkey
- Expected to export to Philippines
2024
- Began exporting to Chile
2023
- Launched Dermal guard sheet
- FINATREE brand has entered ‘Coupang’ Online store
2022
- Began exporting to Indonesia
- Curovet Atoma-X Launched to Japan
- Launched FINATREE brand and opened an Online store (Naver Smart Store)
2021
- Launched I-Spray
- Launched Skin AtoZ
- DWA, Neo HC Gel obtained CE certification
2020
- Rebraded Curovet, a line of veterinary medical devices
- NDA Plus, Neo Mucosal Forte obtained CE certification
2019
- Acquired Halal Certificate
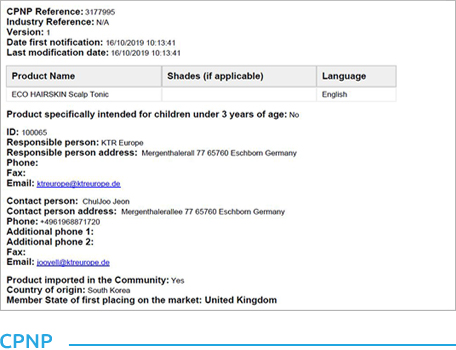
- Obtained CPNP cosmetic certification
- Acquired Class 2 medical device DWA, Neo Mucosal Forte certification
2018
- Acquired ISO 22716 certification
- Began exporting to Vietnam
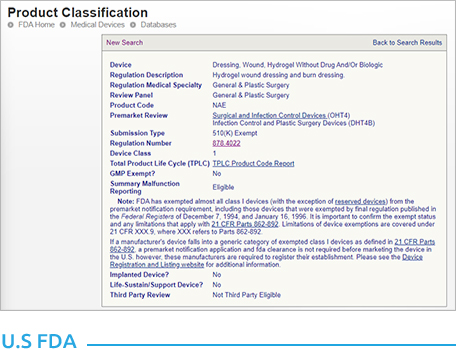
- All medical device products registered in the U.S. FDA
- Eco Hairskin, Eco Clean product registered in CFDA in China
- Relocated Daeun Medical factory
- Launched cosmetic, veterinary medical device product line
- Skin scar treatment composition registered as a patent
2017
- Acquired ISO 13485 certification
- Acquired ISO 9001 certification
- Applied for a patent for hyaluronic acid wound treatment
- Launched Neo Skin-D product
2016
- Patent registration of collagen-containing wound treatment composition
- Launched Neo Mucosal Activator, Neo HC Gel, Eco Shield, Eco AT Gel, Scicosal products
- Launched Neo Dermal Activator product
2010
- Established Daeun Medical factory




Certificates
Daeun Medical is flexible and active in responding to rapidly evolving medical device market changes,
Focusing on research and developing scar treatment products that can be applied to various parts of
life, and making efforts to enter a wider global market.






C.I
Based on the strong and heavy font and color, the blue flame design expresses the meaning of
treating various scars in all life with customized scar treatment materials using high
concentrations of natural plant extracts,
showing the infinite possibility and trust of Daeun Medical.




